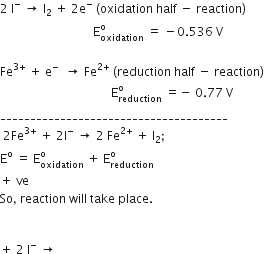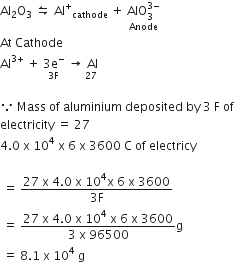A button cell used in watches functions as following
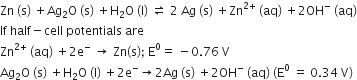
The cell potential will be
-
1.10 V
-
0.42 V
-
0.84 V
-
1.34 V
A.
1.10 V
Anode is always the site of oxidation thus anode half cell is
Zn2+ (aq) +2e- --> Zn (s); E0 =-0.76 V
Cathode half cell is
Ag2O (s) +H2O (l) +2e- ---> 2Ag(s) +2OH- (aq); E0 =0.34 V
E0cell = E0cathode -E0anode
= 0.34 -(-0.76) = +1.10 V