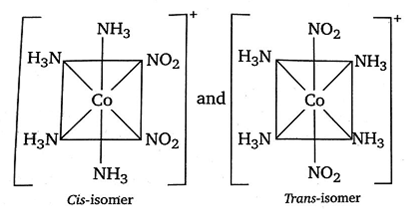
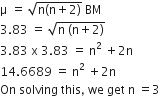[Co(NH3)4(NO2)2]Cl exhibits:
-
linkage isomerism, ionisation isomerism and optical isomerism
-
Linkage isomerism, ionisation isomerism and geometrical isomerism
-
ionization isomerism, geometrical isomerism and optical isomerism
-
linkage isomerism, geometrical isomerism and optical isomerism
B.
Linkage isomerism, ionisation isomerism and geometrical isomerism
The compound [Co(NH3)4((NO2)2]Cl exhibits linkage, ionisation and geometrical isomerism.
Hence, its linkage isomers are
(i) [Co(NH3)2(NO2)2]Cl and [Co(NH3)4(ONO)2]Cl
(ii) Its ionisation isomers are
[Co(NH3)4 (NO2)Cl]NO2 and [Co(NH3)4(NO2)2]Cl
(iii) Its geometrical isomers are