Thermodynamics
Sponsor Area
A Carnot engine operating between temperatures T1 and T2 has efficiency 1/6. When T2 is lowered by 62 K, its efficiency increases to 1/3. Then T1 and T2 are, respectively
-
372 K and 330 K
-
330 K and 268 K
-
310 K and 248 K
-
372 K and 310 K
D.
372 K and 310 K
The efficiency is given by,
Sponsor Area
A Carnot engine, having an efficiency of η = 1/10 as heat engine, is used as a refrigerator. If the work done on the system is 10 J, the amount of energy absorbed from the reservoir at lower temperature is
-
99 J
-
90 J
-
1 J
-
100 J
B.
90 J
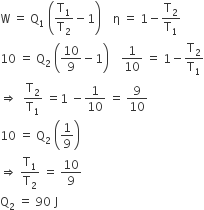
A Carnot engine, whose efficiency is 40%, takes in heat from a source maintained at a temperature of 500 K It is desired to have an engine of efficiency 60%. Then, the intake temperature for the same exhaust (sink) temperature must be
-
the efficiency of Carnot engine cannot be made larger than 50%
-
1200 K
-
750 K
-
600 K
C.
750 K
Efficiency
A diatomic ideal gas is used in a Carnot engine as the working substance. If during the adiabatic expansion part of the cycle the volume of the gas increases from V to 32 V,the efficiency of the engine is
-
0.5
-
0.75
-
0.99
-
0.25
B.
0.75

A solid body of constant heat capacity 1 J/°C is being heated by keeping it in contact with reservoirs in two ways:
(i) Sequentially keeping in contact with 2 reservoirs such that each reservoir supplies the same amount of heat.
(ii) Sequentially keeping in contact with 8 reservoirs such that each reservoir supplies the same amount of heat. In both the cases body is brought from an initial temperature 100°C to final temperature 200°C. Entropy change of the body in the two cases respectively is:
-
ln2,4ln2
-
ln2,ln2
-
ln2,2ln2
-
2ln2,8ln2
B.
ln2,ln2
Since entropy is a state function, therefore a change in entropy in both the processes must be same .
Sponsor Area
Mock Test Series
Mock Test Series





