Redox Reactions
Sponsor Area
Consider the following reaction,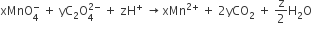
The values of x,y and z in the reaction are respectively
-
5,2 and 16
-
2,5 and 8
-
2,5 and 16
-
5,2 and 8
C.
2,5 and 16
Sponsor Area
Which of the following chemical reactions depicts the oxidizing behaviour of H2SO4?
-
2HI+ H2SO4 →I2 +SO2+2HO
-
Ca(OH)2 +H2SO4 → CaSO4 +2H2O
-
NaCl +H2SO4 → NaHSO4 + HCl
-
2PCl5 +H2SO4 → 2POCl3 + 2HCl + SO2Cl2
A.
2HI+ H2SO4 →I2 +SO2+2HO
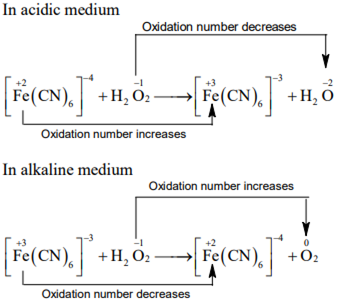
Hydrogen peroxide oxidises in acidic medium but reduces in alkaline medium. The other products formed are, respectively
H2O and (H2O + OH-)
(H2O + O2) and H2O
(H2O + O2) and (H2O + OH-)
H2O and (H2O + O2)
D.
H2O and (H2O + O2)
Sponsor Area
Mock Test Series
Mock Test Series





