Organic Chemistry – Some Basic Principles And Techniques
Sponsor Area
Amount of oxalic acid present in a solution can be determined by its titration with KMnO4 solution in the presence of H2SO4. The titration gives unsatisfactory result when carried out in the presence of HCl, because HCl
-
gets oxidised by oxalic acid to chlorine
-
furnishes H+ions in addition to those from oxalic acid
-
reduces permanganate to Mn2+
-
oxidises oxalic acid to carbon dioxide and water
C.
reduces permanganate to Mn2+
HCl being stronger reducing agent reduces MnO4− to Mn2+ and result of the titration becomes unsatisfactory.
Sponsor Area
An organic compound having molecular mass 60 is found to contain C = 20%, H = 6.67% and N = 46.67% while rest is oxygen. On heating, it gives NH3 along with a solid residue. The solid residue give violet colour with alkaline copper sulphate solution. The compound is
-
CH3NCO
-
CH3CONH2
-
(NH2)2CO
-
CH3CH2CONH2
C.
(NH2)2CO
CH3Br + Nu- → CH3 -Nu +Br-
The decreasing order of the rate of the above reaction with nucleophiles (Nu–) A to D is
[Nu–=
(A) PhO–
(B) AcO–
(C) HO–
(D) CH3O–
]
-
D > C > A > B
-
D > C > B > A
-
A > B > C > D
-
B > D > C > A
A.
D > C > A > B
Due to the presence of an unpaired electron, free radicals are:
-
Chemically reactive
-
Chemically inactive
-
Anions
-
Cations
A.
Chemically reactive
For which of the following molecule significant μ≠ 0?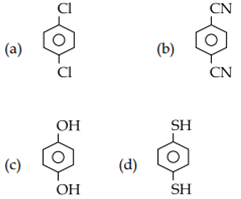
-
only a
-
a and b
-
Only c
-
c and d
D.
c and d
Sponsor Area
Mock Test Series
Mock Test Series





