A PHP Error was encountered
Severity: Notice
Message: Undefined variable: temp_qds
Filename: competition/Study_Question.php
Line Number: 141
Backtrace:
File: /home/wiredfa1/public_html/application/views/final/competition/Study_Question.php
Line: 141
Function: _error_handler
File: /home/wiredfa1/public_html/application/controllers/Competition.php
Line: 339
Function: view
File: /home/wiredfa1/public_html/index.php
Line: 315
Function: require_once
Electrochemistry
Sponsor Area
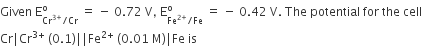
-
0.26
-
0.399
-
0-399
-
-0.26
0.26
0.399
0-399
-0.26
A.
0.26

Sponsor Area
Aluminium oxide may be electrolysed at 1000o C to furnish aluminium metal (Atomic mass = 27 amu; 1 Faraday = 96,500 Coulombs). The cathode reaction is
Al3+ +3e- → Alo
To prepare 5.12 kg of aluminium metal by this method would require
-
5.49 x 107 C of electricity
-
1.83 x 107 C of electricity
-
1.49x 107 C of electricity
-
5.49 x 101 C of electricity
A.
5.49 x 107 C of electricity
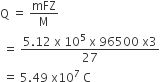
Calomel (Hg2Cl2) on reaction with ammonium hydroxide gives
-
HgNH2Cl
-
NH2 – Hg – Hg – Cl
-
Hg2O
-
HgO
A.
HgNH2Cl
Hg2Cl2 + 2NH4OH → Hg + Hg(NH2)Cl + NH4Cl + 2H2O
Consider the following E° values
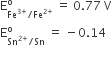
Under standard conditions the potential for the reaction
Sn(s) + 2Fe3+(aq) → 2Fe2+(aq) + Sn2+(aq) is
-
1.68 V
-
0.63 V
-
0.91 V
-
1.40 V
C.
0.91 V
Four successive members of the first-row transition elements are listed below with atomic numbers. Which one of them is expected to have the highest ![]() value?
value?
-
Cr (Z =24)
-
Mn(Z =25)
-
Fe (Z = 26)
-
Co (Z= 27)
D.
Co (Z= 27)
Sponsor Area
Mock Test Series
Mock Test Series





