Aldehydes, Ketones And Carboxylic Acids
Sponsor Area
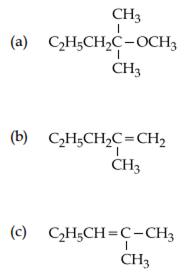
2-chloro-2-methylpentane on reaction with sodium methoxide in methanol yields:
-
Both a and c
-
Only c
-
Both a and b
-
All of these
D.
All of these
Strong nucleophile ![]() polar solvent (MeOH) gives elimination products Products over substitution products but all products are possible in different yields.
polar solvent (MeOH) gives elimination products Products over substitution products but all products are possible in different yields.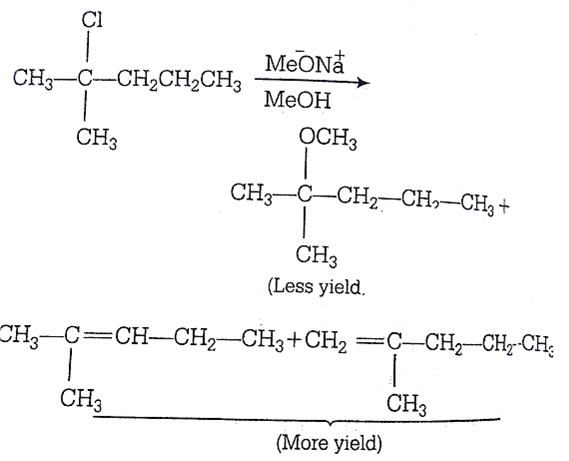
Sponsor Area
Among the following the one that gives positive iodoform test upon reaction with I2 and NaOH is
-
CH3CH2CH(OH)CH2CH3
-
C6H5CH2CH2OH
-
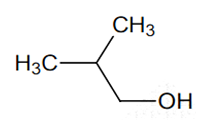
-
PhCHOHCH3
D.
PhCHOHCH3
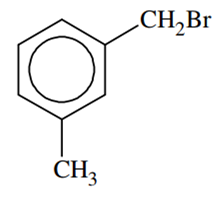
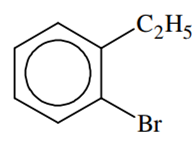
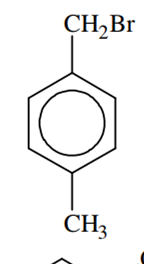
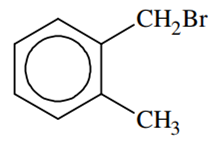
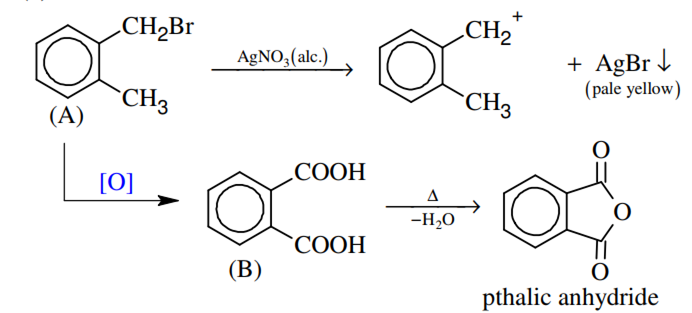
Compound (A), C8H9Br, gives a white precipitate when warmed with alcoholic AgNO3. Oxidation of (A) gives a acid (B), C8H6O4. (B) easily forms anhydride on heating. Identify the compound (A)
D.
In Cannizzaro reaction given below ![]() the slowest step is
the slowest step is
-
The attack of
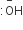 at the carboxyl group
at the carboxyl group -
The transfer of hydride to the carbonyl group
-
The abstraction of proton from the carboxylic group
-
The deprotonation of PhCH2OH
B.
The transfer of hydride to the carbonyl group
Hydride transfer is the slowest step.
In the following sequence of reaction,
![]()
The Product C is
-
C6H5COOH
-
C6H5CH3
-
C6H5CH2OH
-
C6H5CHO
D.
C6H5CHO
Toluene undergoes oxidation with KMnO4, forms benzoic acid. In this conversation, alkyl part of toluene converts into the carboxylic group. Further, the benzoic acid reacts with thionyl chloride (SOCl2) to give benzoyl chloride which upon reduction wth H2/Pd or BaSO4 forms benzaldehyde (Rosenmund reduction).
The reactions are
Sponsor Area
Mock Test Series
Mock Test Series