Sponsor Area
Study Of Gas Laws
What will be the minimum pressure required to compress 500 dm3 of air at 1 bar to 200 dm3 temperature remaining constant.
V1 = 500 dm3
P1 = 1 bar
T1 = 273 K
V2 = 500 dm3
T2 = 273 K
P2= ?
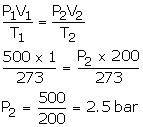
2 litres of a gas is enclosed in a vessel at a pressure of 760 mmHg. If temperature remains constant, calculate pressure when volume changes to 4 dm3.
V = 2 litres
P = 760 mm
V1 = 4000 m3 [1 dm3 = 4 litres]
P1= ?
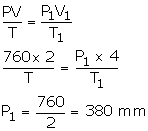
At constant temperature, the effect of change of pressure on volume of a gas was as given below:
|
Pressure in atmosphere |
Volume in litres |
|
0.20 |
112 |
|
0.25 |
89.2 |
|
0.40 |
56.25 |
|
0.60 |
37.40 |
|
0.80 |
28.10 |
|
1.00 |
22.4 |
a. Plot the following graphs
- Pvs V
- Pvs 1/V
- PVvs P
Interpret each graph in terms of a law.
b. Assuming that the pressure values given above are correct, find the correct measurement of the volume.
a.
P/atm | V/dm3 | 1/V | PV |
0.2 | 112 | 0.009 | 22.4 |
0.25 | 89.2 | 0.011 | 22.4 |
0.4 | 56.25 | 0.018 | 22.4 |
0.6 | 37.4 | 0.027 | 22.4 |
0.8 | 28.1 | 0.036 | 22.4 |
1 | 22.4 | 0.045 | 22.4 |
i) P vs. V:
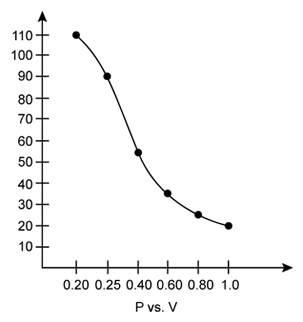
At constant temperature, P is inversely proportional to V. Thus, the plot of V versus P will be a rectangular hyperbola.
ii) P vs. 1/V:
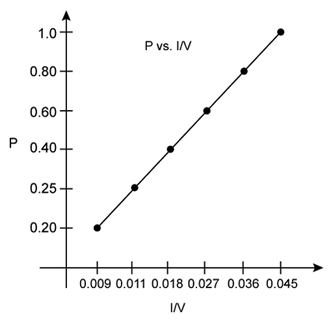
According to Boyle's law, at constant temperature, pressure of a fixed amount of gas varies inversely to its volume. The graph of pressure verses 1/V shows a positive slope.
iii. PV vs. P:
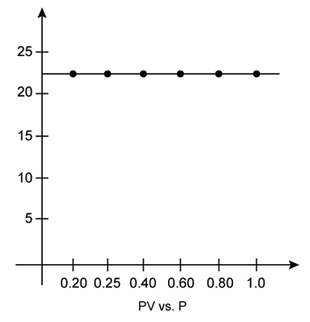
According to Boyle's law, the product of pressure and volume is constant at constant temperature. The graph of PV versus P is constant which indicates that the given gas obeys Boyle's law.
b. The correct measurements of the volume are given below:
P/atm | V/dm3 |
0.2 | 112 |
0.25 | 89.6 |
0.4 | 56 |
0.6 | 37.33 |
0.8 | 28 |
1 | 22.4 |
800 cm3 of gas is collected at 650 mm pressure. At what pressure would the volume of the gas reduce by 40% of its original volume, temperature remaining constant?
Given:
V = 800 cm3
P = 650 m
P1= ?
V1 = reduced volume = 40% of 800
= ![]()
Net V1 = 800 - 320 = 480 cm3
T = T1
Using the gas equation,
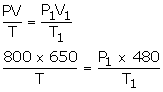
Since T = T1
![]()
Sponsor Area
Mock Test Series
Mock Test Series





