Sponsor Area
Radioactivity
Half life of a certain radioactive substance is 6 hours. If you had 3.2 kg of this substance in the beginning, how much of it will disintegrate in one day?
Using the formula of radioactive decay, 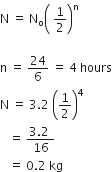
(i) What is the significance of binding energy per nucleon of nucleus?
(ii) In a certain star, three alpha particles undergo fusion in a single reaction to form 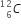 nucleus. Calculate the energy released in the reaction in MeV.
nucleus. Calculate the energy released in the reaction in MeV.
(i) Binding Energy is the total binding energy of nucleus divided by number of nucleons inside it.
Higher the average binding energy per nucleon, greater is the stability of nucleus.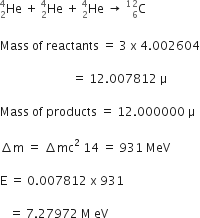
What is the essential difference between the working of a nuclear reactor and that of a fission bomb?
In a nuclear reactor energy released is controlled that is by the use of neutron absorbing material such as Cadmium, where as in a fission bomb their is no control in the amount of energy released.
Complete the following table for a radioactive element whose half life is 5 minutes. Assume that you have 32 g of this element at start, i.e. at t = 0.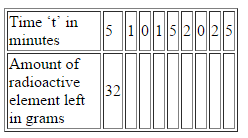
Now, using this data, plot the “decay curve”.
b)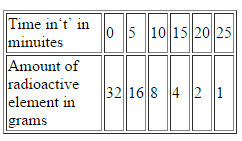
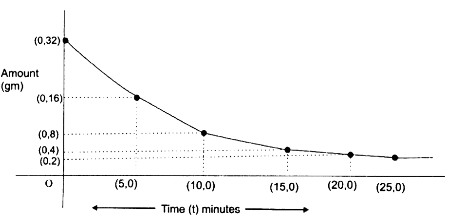
Sponsor Area
Mock Test Series
Mock Test Series





