Give balanced chemical equation for the following conversion A, B and C:
A: 2Fe +3Cl2 -->2FeCl3
B:FeCl3 +Na2CO3 --> FeCO3 +NaCl
C:FeCO3 +2HNO3 --> Fe(NO3)2 +H2O+CO2Sponsor Area
Give balanced chemical equation for the following conversion A, B and C:
A: 2Fe +3Cl2 -->2FeCl3
B:FeCl3 +Na2CO3 --> FeCO3 +NaCl
C:FeCO3 +2HNO3 --> Fe(NO3)2 +H2O+CO2Sponsor Area
Fill in the blanks from the choice given within bracket:
The basicity of Acetic Acid is ……… (3,1,4).
Answer:- 1
Draw the structure of the stable positive ion formed when an acid dissolves in water.
Hydronium ion (H3O+)
H+ +H2O ----> H3O+
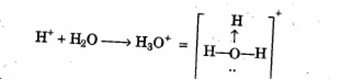
State the inference drawn from the following observations:
(iii) pH of liquid R is 10. What kind of substance is R?
(iv) Salt S is prepared by reacting dilute sulphuric acid wit copper oxide. Identify S.
i) Since the pH is 10, then the substance is the base.
ii) S: Copper sulphate (CuSO4)
Sponsor Area
Mock Test Series