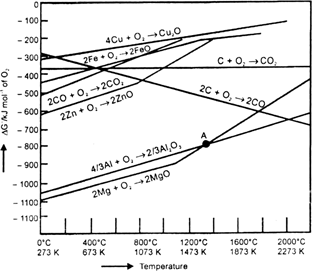
Which of the ores mentioned in Table 6.1. could be concentrated by magnetic separation method?
Table 6.1 Principal ores of some important metal.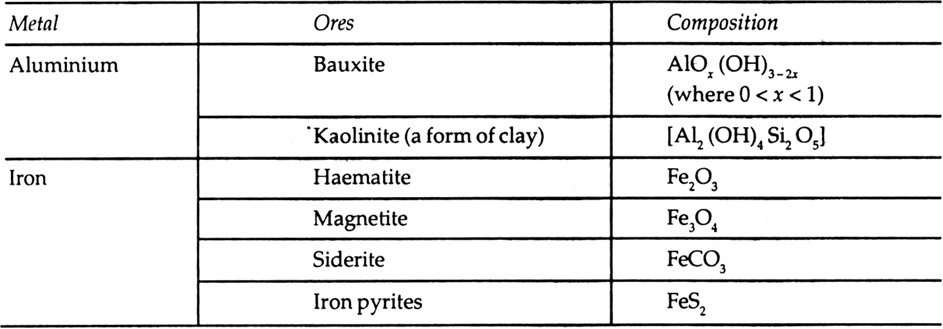

The ore which can be attracted by the magnetic field can be concentrated by the process of magnetic separation. Among the ores mentioned in table 6.1, the ores of iron such as haematite (Fe2O3), magnetite (Fe3O4), siderite (FeCO3), and iron pyrites (FeS2) can be separated by the process of magnetic separation.