Explain hyperconjugation effect or no-bond resonance.
This effect is also called anchimeric effect or Balker Nathan effect.
This effect is explained on the basis of:
(i) molecular orbital concept and
(ii) resonance effect.
(iii) Explanation of the molecular orbital concept. Hyperconjugation is the derealization of the electrons brought about by the sideway overlapping of the p-orbitals of the double bond and σ; orbital of the C – H bond of the alkyl group pz electrons of the double bond and (sp3 – s) σ;-orbital of C – H bond. For example, let us consider the delocalisation of electrons in the alkene ![]() where
where ![]() represents an alkyl group. The delocalisation may be shown as:
represents an alkyl group. The delocalisation may be shown as: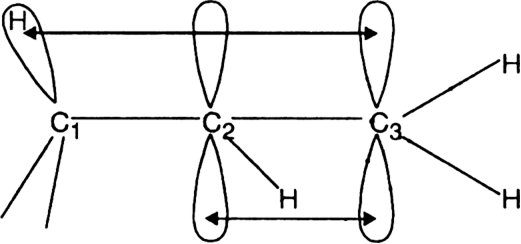
Thus electron pair forming C – H bond of the  -carbon not only binds these atoms (C and H) together but also binds the two carbon atoms of the double bond to some extent. Similarly, the electron pair forming the
-carbon not only binds these atoms (C and H) together but also binds the two carbon atoms of the double bond to some extent. Similarly, the electron pair forming the  ;-bond not only binds these doubly bonded carbon atoms together but also binds the carbon and hydrogen atoms to some extent. In other words, delocalisation helps in bonding together all the four atoms to some extent i.e. there are four electrons delocalised over four nuclei (C1 C2, C3 and H).
;-bond not only binds these doubly bonded carbon atoms together but also binds the carbon and hydrogen atoms to some extent. In other words, delocalisation helps in bonding together all the four atoms to some extent i.e. there are four electrons delocalised over four nuclei (C1 C2, C3 and H).
(ii) Explanation of resonance effect. According to this concept, if an alkyl group carrying at least one hydrogen is attached to an unsaturated carbon atom, it releases the electrons of C–H bond towards the multiple bonds. For example, propylene can be considered to be resonance hybrid of the following four structures: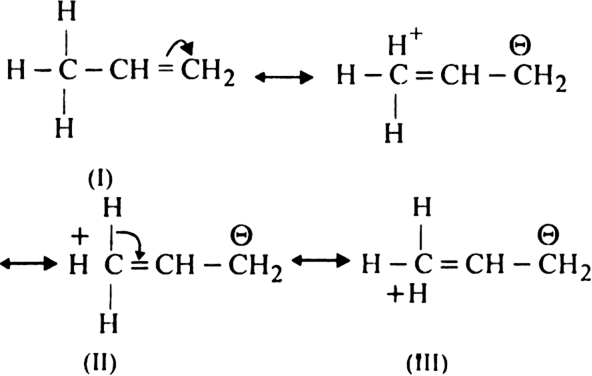
Structures I, II and III are hyperconjugation structures. Since there is no bond between carbon and hydrogen atom in these structures, hyperconjugation is also called no-bond resonance. Therefore, more the number of such  -hydrogen atoms, more are the number of hyperconjugation structures and hence greater is the inductive effect. The number of hydrogen atoms is three with the methyl group, two with the ethyl group, one with the isopropyl group and none with the tertbutyl group. Thus, the order of hyperconjugation effect decreases in the order:
-hydrogen atoms, more are the number of hyperconjugation structures and hence greater is the inductive effect. The number of hydrogen atoms is three with the methyl group, two with the ethyl group, one with the isopropyl group and none with the tertbutyl group. Thus, the order of hyperconjugation effect decreases in the order:
CH3 – > CH3CH2 – > (CH3)2CH – > (CH3)3C –







 - electron system because of hyperconjugation. Carbon is slightly more electronegative than hydrogen. Thus, the carbon atom in an alkyl group has higher electron density around it as compared with an H atom. As a result, alkyl group are able to donate electrons inductively when attached to a pi system. Let us illustrate this by taking an example of propylene. The various resonating structures are as follows:
- electron system because of hyperconjugation. Carbon is slightly more electronegative than hydrogen. Thus, the carbon atom in an alkyl group has higher electron density around it as compared with an H atom. As a result, alkyl group are able to donate electrons inductively when attached to a pi system. Let us illustrate this by taking an example of propylene. The various resonating structures are as follows: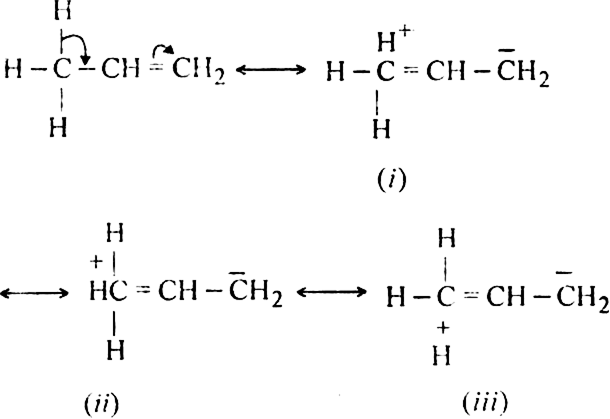
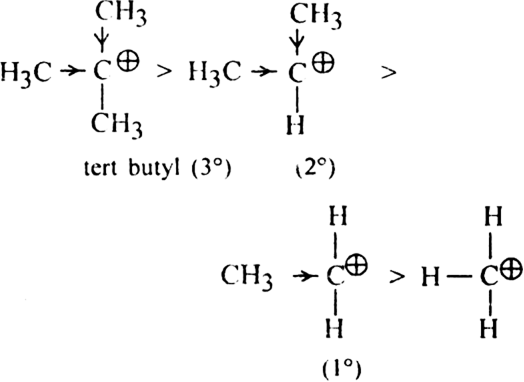
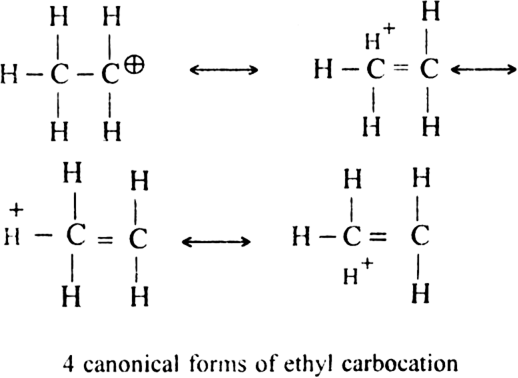
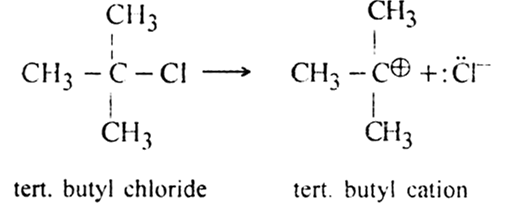
 are two simple carbocations known as methyl cation and ethyl cations respectively? Carbocations are very short-lived and highly reactive species. Among primary (1°), secondary (2°) and tertiary (3°) carbocations, 3° is most stable.
are two simple carbocations known as methyl cation and ethyl cations respectively? Carbocations are very short-lived and highly reactive species. Among primary (1°), secondary (2°) and tertiary (3°) carbocations, 3° is most stable.