According to Bohr's theory:
(i) The atom consists of a small (positively charged) nucleus at its centre.
(ii) The whole mass of the atom is concentrated in the nucleus and the volume of the nucleus is smaller than the volume of the atom by a ratio of about 1 : 105.
(iii) All the protons and neutrons of the atom are contained in the nucleus.
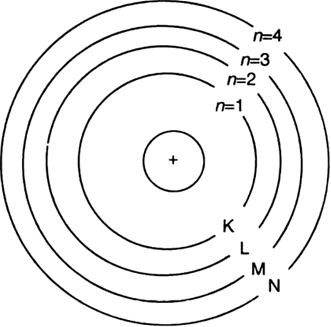
(iv) The electrons of the atom revolve around the nucleus in definite circular paths known as orbits or which are designated as K, L, M, N etc. or numbered as (n) = 1, 2, 3, 4 etc. outward from the nucleus.
(v) Each orbit is associated with a fixed amount of energy. Therefore, these orbits are also known as energy levels or energy shells.
(vi) The energy of the atom changes when an electron jumps from one state (energy level) to another state (energy level). As long as an electron remains in a particular orbit, it does not lose or gain energy.






