Sponsor Area
Atoms
A proton and an electron travelling along parallel paths enter a region of uniform magnetic field, acting perpendicular to their paths. Which of them will move in a circular path with higher frequency ?
Mass of electron is low as compared to proton. Hence when both enter into the uniform magnetic region, the electron will move in a circular path with higher frequency on the opposite direction to the current.
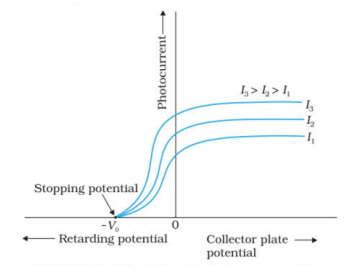
Draw graphs showing a variation of the photoelectric current with applied voltage for two incident radiations of equal frequency and different intensities. Mark the graph for the radiation of higher intensity.
Four nuclei of an element undergo fusion to form a heavier nucleus, with the release of energy. Which of the two — the parent or the daughter nucleus — would have higher binding energy per nucleon?
Daughter nuclei are more stable than parent nuclei.
State Bohr’s postulate to define stable orbits in hydrogen atom. How does de Broglie’s hypothesis explain the stability of these orbits?
Bohr's postulate:
An atom has a number of stable orbits in which an electron can reside without the emission of radiant energy. Each orbit corresponds, to a certain energy level.
Electrons revolve in a circular orbit. Centripetal force is provided by electrostatic force between electron and proton.
Given as,
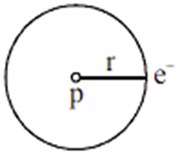
The motion of an electron in a circular orbit is restricted in such a manner that its angular momentum is an integral multiple of h/2π, Thus
3.
An electron may jump spontaneously from one orbit (energy level E1) to the other orbit (energy level E2) (E2 > E1); then the energy change AE in the electron jump is given by Planck’s equation
∆E = E2-E1 = hv
Where h = Planck’s constant.
And v = frequency of light emitted.
When the electron jumps from nth higher orbit to pth lower orbit it emits energy in form of the photon.
En - Ep = hv
According to de'Broglie hypothesis
Sponsor Area
Mock Test Series
Mock Test Series





