Sponsor Area
Structure of Atom
Calculate the wavelength of an electron moving with a velocity of 2.05x 107 ms-1.
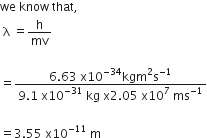
Name the fundamental particles which constitute an atom.
Fundamental particles which constitute an atom are electrons, protons and neutrons.
What is an Electron?
Electron is a subatomic particle having a unit negative charge (-1.602 x 10-19coulombs) and a mass (9.11 x 10-31 kg) which is 1/1837 of that of a hydrogen atom.
What is a proton?
Proton is a subatomic particle of an atom having a unit positive charge (1.602x10-19 coulombs) and a mass (1.67x 10-27 kg) which is practically the same as that of a hydrogen atom.
Sponsor Area
Mock Test Series
Mock Test Series





