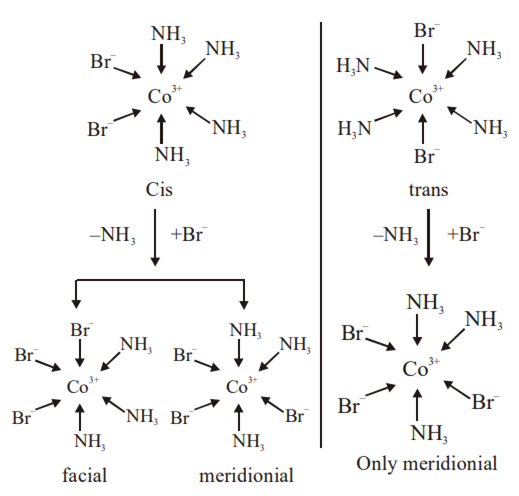
Consider the following reaction and statements:
(I) Two isomers are produced if the reactant complex ion is a cis-isomer
(II) Two isomers are produced if the reactant complex ion is a trans-isomer
(III) Only one isomer is produced if the reactant complex ion is a trans-isomer
(IV) Only one isomer is produced if the reactant complex ion is a cis – isomer
The correct statements are
(II) and (IV)
(I) and (II)
(I) and (III)
(III) and (IV)
C.
(I) and (III)