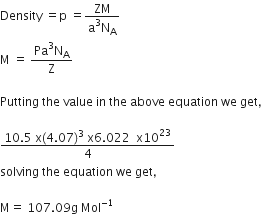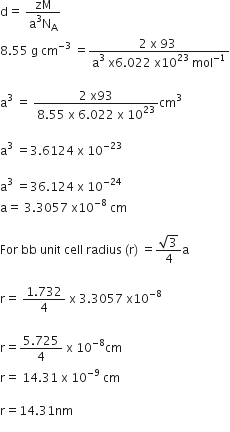How would you account for the following:
Schottky defects lower the density of related solids.
It is basically a vacancy defect in ionic solids. In order to maintain electrical neutrality, the number of missing cations and anions are equal.
Thus there is equal number of positive and negtive ion leave correct lattice point and go outside the lattice creating a pair of vacancy. So there are less number of ions thannbefore whichresult in the decrease in its density.