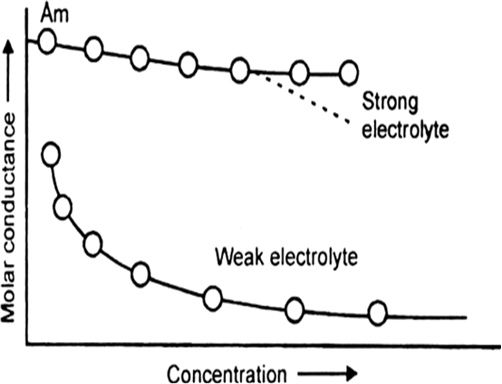
How does molar conductivity vary with concentration for (i) weak electrolyte and for (ii) strong electolyte? Give reasons for these variations.
(i) Weak electrolytes: When the concentration of weak electrolyte becomes very low, its degree of ionisation rises sharply. There is sharp increase in the number of ions in the solution. Hence, the molar conductivity of a weak electrolyte rises steeply at low concentration.
(ii) Strong electrolytes: The molar conductivity of a strong electrolyte decreases slightly with the increase in concentration. This decrease is due to the increase in interionic attractions as a result of greater numbr of ions per unit volume. With dilution, the ions are far apart, inter ionic attractions become weaker and conductance increases.