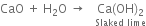Give one method of preparing quick lime. What happens when rain water falls on it?
Calcium oxide is called quicklime. It is prepared by heating the lime stone in a rotatory kiln at 1070-1270 K.
CO2 formed escapes and hence the above equilibrium shifts towards the formation of calcium oxide.
When rain-water falls on quick lime, slaked lime is formed.