Can we prepare potassium bicarbonate by Solvay process?
Potassium bircarbonate which is to be formed in the process is highly soluble in water and cannot be precipitated.
Can we prepare potassium bicarbonate by Solvay process?
Potassium bircarbonate which is to be formed in the process is highly soluble in water and cannot be precipitated.
What is the action of heat on Na2CO3.10H2O?
On heating below 373K, it loses 9 molecules of water of crystallisation to form monohydrate (Na2CO3.H2O). On heating above 373K, the monohydrate changes to an anhydrous white powder called soda ash but does not decompose further.
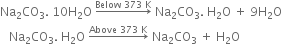
State as to why a solution of Na2CO3 is alkaline.
Na2CO3 is a salt of strong base (NaOH) and weak acid (H2CO3). When Na2CO3 is dissolved in water, it undergoes hydrolysis to produce strong base (NaOH) and weak acid (H2CO3). Hence its aqueous solution is alkaline in nature.
What happens when:
(i) sodium carbonate reacts with the milk of lime.
(ii) sodium carbonate is added to water.
(iii) sodium carbonate reacts with a dilute mineral acid?
(i) It reacts with hot milk of lime to form sodium hydroxide .
.
(ii) It undergoes hydrolysis to form an alkaline solution.
 .
.
(iii) It reacts with dilute mineral acid evolving CO2 gas.  .
.
Sponsor Area
Mock Test Series