Calculate the mass of sodium acetate (CH3COONa) required to make 500 mL of 0·375 molar aqueous solution. Molar mass of sodium acetate is 82·0245g mol–1.
1000 mL of the solution contain sodium acetate = 0·375 mole,
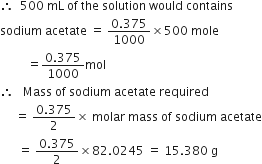
Calculate the mass of sodium acetate (CH3COONa) required to make 500 mL of 0·375 molar aqueous solution. Molar mass of sodium acetate is 82·0245g mol–1.
1000 mL of the solution contain sodium acetate = 0·375 mole,
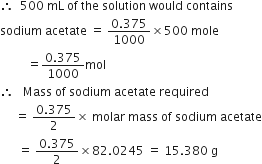
The density of 3M solution of NaCl is 1.25g ml-1. Calculate molality of the solution.
![]()
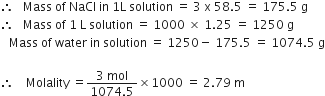
In a reaction vessel 0.184 g of NaOH is required to be added for completing the reaction. How many millilitre of 0.150 M NaOH solution should be added for this requirement?
Molar mass of NaOH = 40 g mol-1![]() 1000 mL of NaOH solution contains 0.150 mol = 0.150 x 40 = 6 g
1000 mL of NaOH solution contains 0.150 mol = 0.150 x 40 = 6 g
It means that 6g sodium hydroxide is present in 1000 mL
![]() 0.184 g sodium hydroxide would be present in
0.184 g sodium hydroxide would be present in
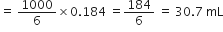
Commercially available concentrated hydrochloric acid contains 38% HCl by mass.
(i) What is the molarity of the solution (density of solution = 1·19g cm-3)?
(ii) What volume of concentrated HCl is required to make 1·0L of 0·10 M HCl?
(i) 38% HCl by mass means that 38g of HCl is present in 100g of solution.
Volume of solution
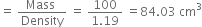
Moles of HCl = 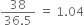
Molarity = 
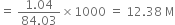
(ii) By applying normality equation,
![]()
Sponsor Area
Mock Test Series