Which of the following aqueous solutions will conduct electricity? Glucose, Hydrochloric acid, Acetic acid, Alcohol, Sulphuric acid, Sodium hydroxide.
Hydrochloric acid, Acetic acid, Sulphuric acid, Sodium hydroxide conduct electricity.
Hydrochloric acid, Acetic acid, Sulphuric acid, Sodium hydroxide conduct electricity.
Take an acid (say HCl) in a beaker. Add water. Dip two graphite rods into it. Complete the circuit as shown below: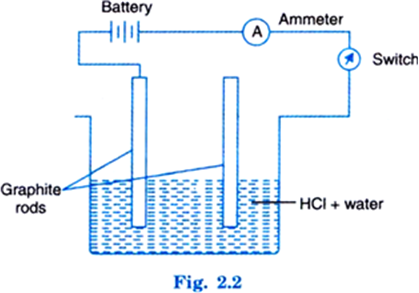
Now switch on the circuit. It will be seen that the pointer of ammeter moves. This shows that current is passing through circuit. This is possible when ions are present in solution.
Now take dry HCl in acetone or any other organic liquid. Arrange the system as before. It will be seen that pointer of the ammeter does not move when circuit is completed. This means no current is passing and solution does not contain ions. Hence, it is inferred that acids produce ions in water solution only.
Electric current flows through the solution by ions. Since dry hydrochloric acid does not give any ions, it does not conduct current. Whereas in the presence of water, H+ ions and CI– ions are produced which are responsible for flow of current.
HCl + H2O → H3+O + Cl–
Tap water conduct electricity because of the presnce of impurities in the forms of salts. These salts produce ion in tap water and thus conduct electricity.
Distilled water is free from all kinds of salts and hence does not conduct electricity.
Sponsor Area
Mock Test Series